Background
…because access to medicine is a fundamental human right


Biologics are produced from living organisms using biotechnology techniques and have tremendous health benefits. They include monoclonal antibodies used in the treatment of cancers and autoimmune disease, hormones, haematopoietic growth factors, interferons and colony stimulating factors.
Unfortunately, many of these are unaffordable to the majority of people and represent a significant burden on the health care systems of countries where they are available. Some of these products are now off-patent and are being produced as biosimilars. Biosimilars are biologics comparable in terms of quality, safety and efficacy to the reference product. The development of biosimilars has greatly helped reduce the cost and availability of medicines.
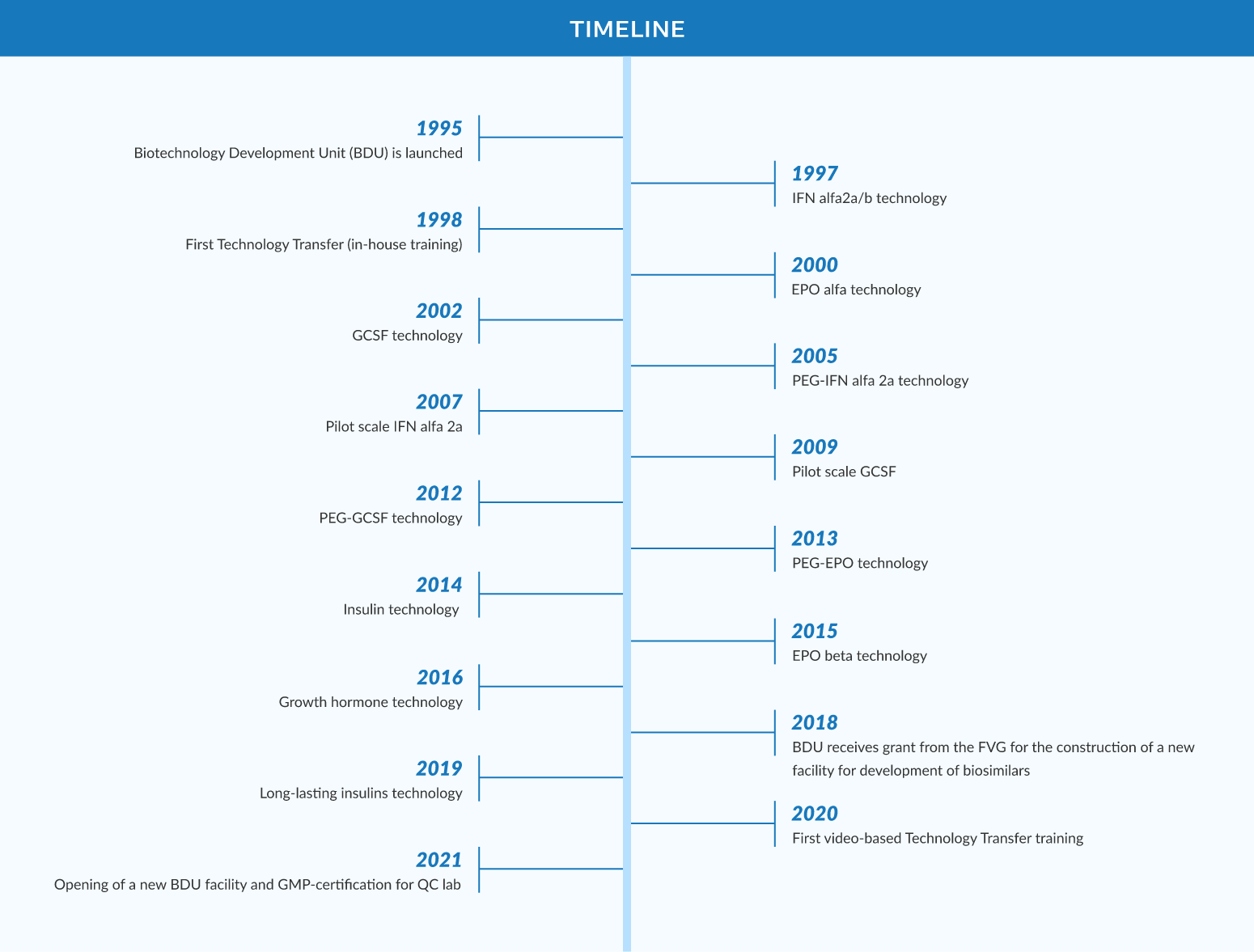
To date, our team has developed strains, protocols and quality control testing for 12 biosimilars. We provide a central facility for high-yield cell lines generation, upstream, downstream and quality control operations plus transfer of know-how to multiple recipients.